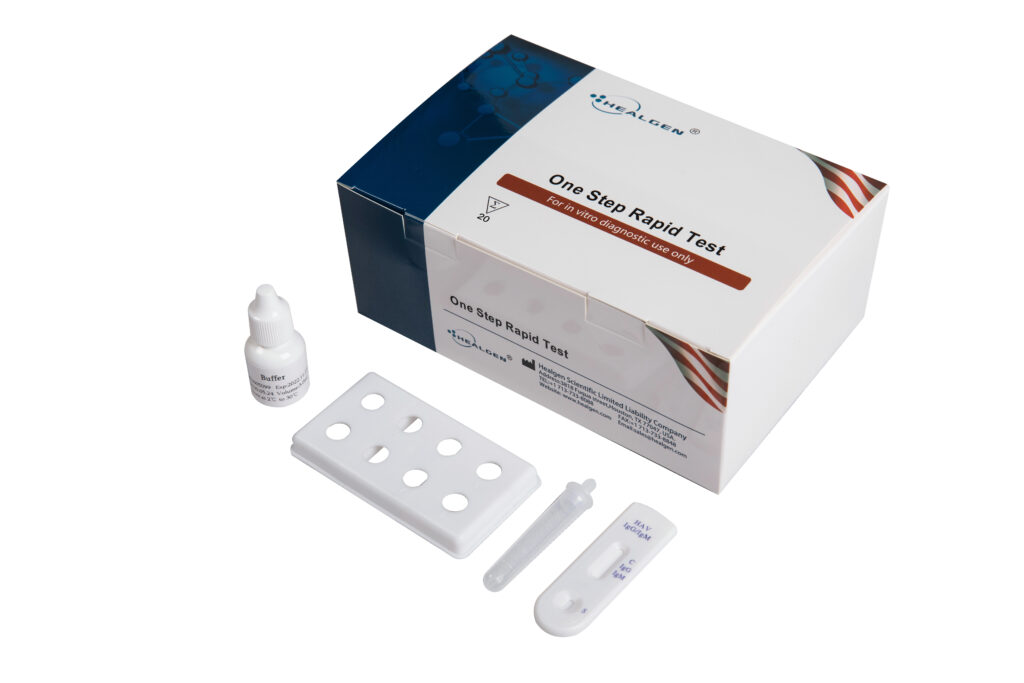
HAV
HAV IgM and IgG Test Cassette is a single use, rapid device intended for qualitative detection of IgM-class and IgG-class antibodies to hepatitis A virus (HAV) in whole blood, serum, plasma or feces samples. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HAV.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Sensitivity: IgM 98.5%
- Specificity: IgM 97.7%
- Specimen: Whole Blood, Serum, Plasma, Feces
- Time to Results: 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| HAV IgM Rapid Test | Serum/Plasma | GCHAV(IgM)- 302Ba | Cassette | 25 Tests/Kit | CE marked |
| HAV IgG/IgM Rapid Test | Whole blood/Serum/Plasma | GCHAV(IgG/IgM)-402a | Cassette | 25 Tests/Kit | CE marked |
| HAV Antigen Rapid Test | Feces | GCHAV-602a | Cassette | 25 Tests/Kit | CE marked |
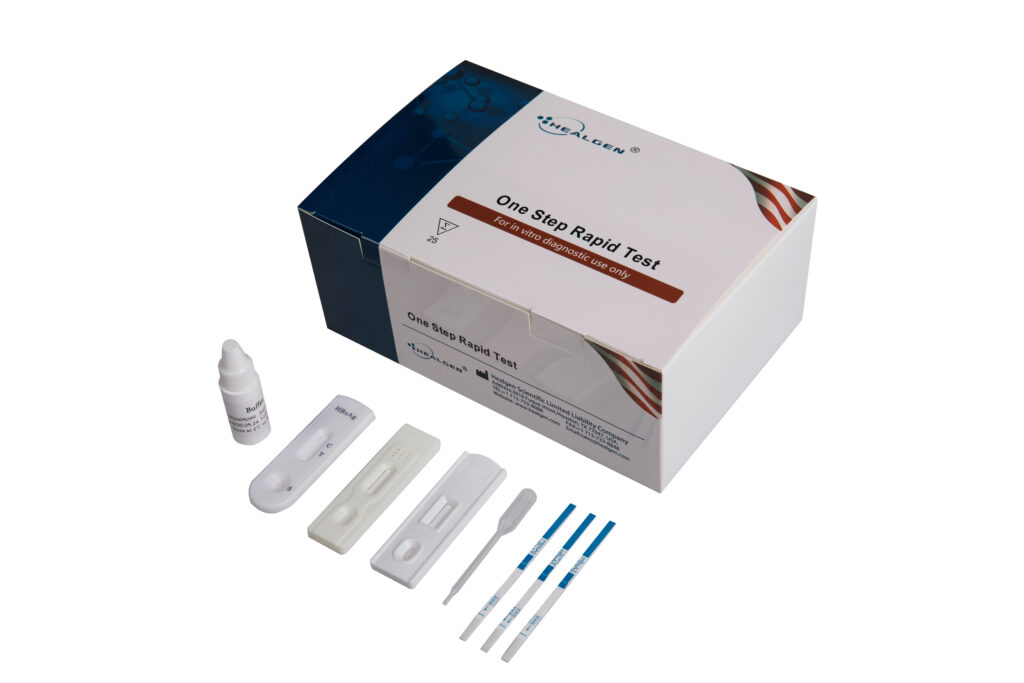
HBV
The Hepatitis B Virus Rapid Tests are rapid chromatographic immunoassays for the qualitative detection of Hepatitis B Surface Antigen (HBsAg), Hepatitis B Surface Antibody (HBsAb), Hepatitis B Envelope Antigen (HBeAg), Hepatitis B Envelope Antibody (HBeAb) and Hepatitis B Core Antibody (HBcAb) in serum, plasma or whole blood.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Sensitivity: HBcAb 96.3%; HBeAb & HbeAg 94.5%; HBsAb 99%; HBsAg 99.4%
- Specificity: HBcAb 96.8%; HBeAb & HbeAg 97.3%; HBsAb 99.56%; HBsAg 99.5%
- Specimen: Whole Blood, Serum, Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Cut-Off Value | Kit Size | Qualification |
|---|---|---|---|---|---|---|
| HBcAb Hepatitis B Core Antibody Test | Serum/Plasma | GCHBcb-302a | Cassette | 2 NCU | 25 Tests/Kit | / |
| GCHBcb-302b | Cassette | 8 NCU | 25 Tests/Kit | / | ||
| Whole blood/Serum/Plasma | GCHBcb-402a | Cassette | 2 NCU | 25 Tests/Kit | / | |
| HBeAb Hepatitis B Envelope Antibody Test | Serum/Plasma | GCHBeb-302a | Cassette | 2 NCU | 25 Tests/Kit | / |
| GCHBeb-302b | Cassette | 8 NCU | 25 Tests/Kit | / | ||
| Whole blood/Serum/Plasma | GCHBeb-402a | Cassette | 2 NCU | 25 Tests/Kit | / | |
| HBeAg Hepatitis B Envelope Antigen Test | Serum/Plasma | GCHBeg-302a | Cassette | 0.5 NCU | 25 Tests/Kit | / |
| Whole blood/Serum/Plasma | GCHBeg-402a | Cassette | 0.5 NCU | 25 Tests/Kit | / | |
| HBsAb Hepatitis B Surface Antibody Test | Serum/Plasma | GCHBsb-301a | Strip | 30 mIU/mL | 50 Tests/Kit | / |
| GCHBsb-302a | Cassette | 30 mIU/mL | 25 Tests/Kit | / | ||
| Whole blood/Serum/Plasma | GCHBsb-401a | Strip | 30 mIU/mL | 50 Tests/Kit | / | |
| GCHBsb-402a | Cassette | 30 mIU/mL | 25 Tests/Kit | / | ||
| GCHBsb-402b | Cassette | 20 mIU/mL | 25 Tests/Kit | / | ||
| HBsAg Hepatitis B Surface Antigen Rapid Test | Serum/Plasma | GCHBsg-301a | Strip | 1 ng/mL | 50 Tests/Kit | / |
| GCHBsg-302a | Cassette | 1 ng/mL | 25 Tests/Kit | / | ||
| Whole blood/Serum/Plasma | GCHBsg-401a | Strip | 1 ng/mL | 50 Tests/Kit | / | |
| GCHBsg-402a | Cassette | 1 ng/mL | 25 Tests/Kit | / |
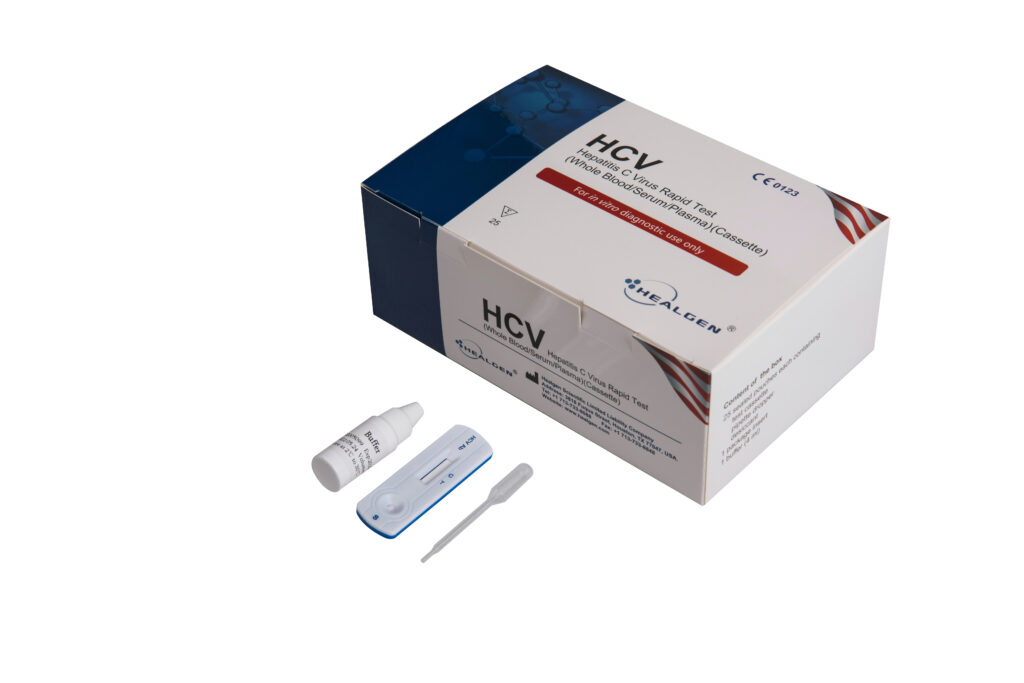
HCV
The Hepatitis C (HCV) Antibody Rapid Test is a sandwich lateral flow chromatographic immunoassay for the qualitative detection of anti-HCV antibodies IgG, IgM, and IgA in human serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of HCV infection.
Features:
- Two band results for simple interpretation
- Detects anti-HCV IgG, IgM and IgA
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
Specifications:
- Sensitivity: 98.1%
- Specificity: 98.9%
- Specimen: Whole Blood, Serum, Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
HCV Hepatitis C Virus Test | Serum/Plasma | GCHCV-301a | Strip | 50 Tests/Kit | / |
| Serum/Plasma | GCHCV-302a | Cassette | 25 Tests/Kit | CE 0123 | |
| Whole blood/Serum/Plasma | GCHCV-401a | Strip | 50 Tests/Kit | / | |
| Whole blood/Serum/Plasma | GCHCV-402a | Cassette | 25 Tests/Kit | CE 0123 |
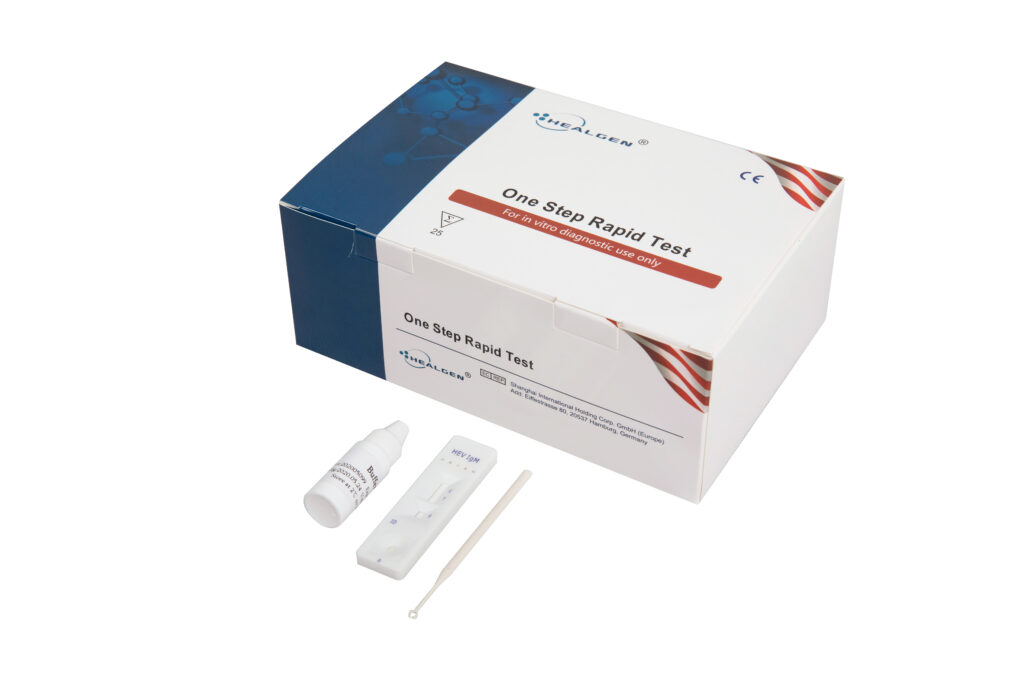
HEV
The Hepatitis C (HCV) Antibody Rapid Test is a sandwich lateral flow chromatographic immunoassay for the qualitative detection of anti-HCV antibodies IgG, IgM, and IgA in human serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of HCV infection.
Features:
- Easy operate and simple interpretation
- Detects anti-HEV IgM
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Sensitivity: >99%
- Specificity: 98.9%
- Specimen: Serum, Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| HEV Hepatitis E Virus Antibody Test | Serum/Plasma | GCHEV-302a√ | Cassette | 25 Tests/Kit | CE marked |
HIV
The Human Immunodeficiency Virus Rapid Tests are rapid chromatographic immunoassays for the qualitative detection of HIV p24 antigen and/or antibodies (IgG, IgM, and IgA) to HIV-1 and/or HIV-2 and/or Subtype O in serum, plasma or whole blood. It is intended to be used as a screening test and as an aid in the diagnosis of infection with HIV.
Features:
- Facilitates patient treatment decisions quickly
- Simple, time-saving procedure
- High sensitivity
- Reliable results
- CE List A certificate obtained
Specifications:
- Sensitivity: >99%
- Specificity: >99%
- Specimen: whole blood, serum or plasma
- Time to Results: 10 – 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| HIV 1/2 Ab Rapid Test | Serum/Plasma | GCHIV-301a | Strip | 50 Tests/Kit | / |
| HIV 1/2 Ab Rapid Test | Serum/Plasma | GCHIV-302a√ | Cassette | 25 Tests/Kit | CE 0123 |
| HIV 1/2 Ab Rapid Test | Whole Blood/Serum/Plasma | GCHIV-401a | Strip | 50 Tests/Kit | / |
| HIV 1/2 Ab Rapid Test | Whole Blood/Serum/Plasma | GCHIV-402a√ | Cassette | 25 Tests/Kit | CE 0123 |
| HIV 1/2 Ab Tri-line Rapid Test | Whole Blood/Serum/Plasma | GCHIV-GT402a | Cassette | 25 Tests/Kit | / |
| HIV 1/2/O Ab Tri-line Rapid Test | Serum/Plasma | GCHIV-T302b | Cassette | 25 Tests/Kit | / |
| HIV 1/2/O Ab Tri-line Rapid Test | Whole Blood/Serum/Plasma | GCHIV-T402a | Cassette | 25 Tests/Kit | / |
| HIV Ab/Ag Rapid Test | Whole Blood/Serum/Plasma | GCHIV(Ag/Ab)-402a | Cassette | 25 Tests/Kit | / |
VISITECT CD4
The VISITECT CD4 Advanced Disease Rapid Test is a manually operated semi-quantitative assay for the estimation of CD4 protein on the surface of CD4+ T cells in human whole blood (capillary or EDTA venous) to indicate whether the level is above or below 200 cells/μL within prediagnosed HIV patients.
Features:
- Rapid estimation CD4+ T cell count levels
- Receive an accurate result within an assay time for just 40 minutes.
- Accelerate clinical diseases management
- Test anywhere, any time
- Reduce patient loss to follow-up
Specifications:
- Capillary samples: Sensitivity: 89.3%; Specificity: 92.3%;
- Venous blood: Sensitivity: 86.3%; Specificity: 92.8%;
- Specimen: whole blood
- Assay time: 40 minutes
- Storge: 2-30℃
- Shelf Life: 12 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| VISITECT CD4 Advanced Disease | Whole blood | OD376 | Cassette | 50 Tests/Kit | CE marked, WHO PQ |
Syphilis (TP)
The Syphilis Ab Rapid Test Cassette uses a combination of syphilis antigen-colloidal gold conjugate and immobilized syphilis antigen to detect T. pallidum antibodies (IgG, IgM and IgA) qualitatively and selectively in whole blood, serum or plasma.
Features:
- Two band results for simple interpretation
- Detects syphilis antibodies IgG ,IgM and IgA
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
- Internal control included
Specifications:
- Sensitivity: 99.54%
- Specificity: 100%
- Specimen: Whole blood/Serum/Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| Syphilis Antibody Rapid Test | Serum/Plasma | GCSYP-301a | Strip | 50 Tests/Kit | CE marked |
| Syphilis Antibody Rapid Test | Serum/Plasma | GCSYP-302a | Cassette | 25 Tests/Kit | CE marked |
| Syphilis Antibody Rapid Test | Whole blood/Serum/Plasma | GCSYP-401a | Strip | 50 Tests/Kit | CE marked |
| Syphilis Antibody Rapid Test | Whole blood/Serum/Plasma | GCSYP-402a | Cassette | 25 Tests/Kit | CE marked |
| HBsAg/HCV/HIV/Syphilis Combo Rapid Test | Serum/Plasma | GCHBCISY-345a | Multi-panel Cassette | 20 Tests/Kit | / |
| HBsAg/HCV/HIV/Syphilis Combo Rapid Test | Whole blood/Serum/Plasma | GCHBCISY-445a | Multi-panel Cassette | 20 Tests/Kit | / |
HBV HBcAb/HBeAb/HBeAg/HBsAb/HBsAg Combo
The Hepatitis B Virus Combo Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid chromatographic immunoassay for the qualitative detection of Hepatitis B surface Antigen (HBsAg), Hepatitis B surface Antibody (HBsAb), Hepatitis B Envelop Antigen (HBeAg), Hepatitis B Envelope (HBeAb), and Hepatitis B Core Antibody (HBcAb) in human whole blood, serum and plasma.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Clinical Evaluations:
- HBsAg: Sensitivity: >99%; Specificity: 96.8%; Accuracy: 98.3%
- HBsAb: Sensitivity: >99%; Specificity: 98.7%; Accuracy: 99.5%
- HBeAg: Sensitivity: 98.2%; Specificity: 98.2%; Accuracy: 98.2%
- HBeAb: Sensitivity: 94.5%; Specificity: 97.3%; Accuracy: 96.6%
- HBcAb: Sensitivity: 96.3%; Specificity: 96.8%; Accuracy: 96.4%
- Specimen: Whole blood/Serum/Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months after the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
HBV HBcAb/HBeAb/HBeAg/HBsAb /HBsAg Combo Test | Serum/Plasma | GCHBV-355a | Cassette | 20 Tests/Kit | / |
| Whole blood/Serum/Plasma | GCHBV-455a | Cassette | 20 Tests/Kit | / |
HBsAg & HCV Combo
The HBsAg & HCV Combo Rapid Test is an immunochromatographic assay for the qualitative detection of HCV antibodies and HBsAg respectively, in human whole blood, serum or plasma samples. It is intended for professional use as an aid in diagnosing HCV, HBsAg infections.
Features:
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Clinical Evaluations:
- HCV: Sensitivity: 98.8%; Specificity: 99.6%; Accuracy: 99.5%
- HBsAg: Sensitivity: 99.5%; Specificity: 99.7%; Accuracy: 99.7%
- Specimen: Whole blood/Serum/Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months after the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| HBsAg/HCV Combo Rapid Test | WB/S/P | GCHBC-402a | Cassette | 25 Tests/Kit | / |
HBsAg/HCV/HIV/Syphilis Combo
The HIV/HCV/HBsAg/Syphilis Combo Rapid Test is a rapid, immunochromatographic assay for the qualitative detection of HCV antibodies, HIV 1/2 virus- antibodies, HBsAg, and/or Treponema Pallidum antibodies respectively, in human whole blood, serum or plasma samples. It is intended for professional use as an aid in diagnosing HCV, HIV, HBV, and/or Syphilis infections. This assay provides only a preliminary result and all positive specimens should be confirmed with other qualified assays.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Internal control included
- Reagents included
Specifications:
- Clinical Evaluations:
- HCV: Sensitivity: 98.1%; Specificity: 98.9%; Accuracy: 98.9%
- HBsAg: Sensitivity: 99.4%; Specificity: 99.5%; Accuracy: 99.5%
- HIV: Sensitivity: 99.5%; Specificity: 99.8%; Accuracy: 99.8%
- Syphilis: Sensitivity: 99.5%; Specificity: 99.7%; Accuracy: 99.7%
- Specimen: Whole blood/Serum/Plasma
- Time to Results: 15 minutes
- Shelf Life: 24 months after the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| HBsAg/HCV/HIV/Syphilis Combo Test | Serum/Plasma | GCHBCISY-345a | Cassette | 20 Tests/Kit | / |
| Whole blood/Serum/Plasma | GCHBCISY-445a | Cassette | 20 Tests/Kit | / |
HCV/HIV Combo
The HIV / HCV Combo Rapid Test Cassette (Whole Blood/Serum/Plasma) is a rapid, immunochromatographic assay for the qualitative detection of HCV antibodies, HIV 1/2 virus- antibodies respectively, in human whole blood, serum or plasma samples. It is intended for professional use as an aid in diagnosing HCV, HIV infections. This assay provides only a preliminary result and all positive specimens should be confirmed with other qualified assays.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Reagents included
Specifications:
- Clinical Evaluations:
- HCV: Sensitivity: 98.1%; Specificity: 98.9%; Accuracy: 98.9%
- HIV: Sensitivity: 99.5%; Specificity: 99.8%; Accuracy: 99.8%
- Specimen: Whole blood/Serum/Plasma
- Time to Results: 10 minutes
- Shelf Life: 24 months after the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
| HCV/HIV Combo Test | Whole blood/Serum/Plasma | GCHCI-402a | Cassette | 25 Tests/Kit | / |
