Rapid COVID-19 Antigen Tests
The Healgen Rapid COVID-19 Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection of SARS-CoV-2 nucleocapsid protein in direct anterior nasal swab specimens from individuals who are suspected of COVID-19 by their healthcare provider within the first seven (7) days of symptom onset.
(For professional in vitro diagnostic use only)
Features:
- Health Canada Medical Device Authorization (# 353562) Class IV IVD for POC professional use.
- Rapid and accurate results within 15 minutes.
- Strong test performance:
Positive Percent Agreement: 84.2%
Negative Percent Agreement: 99.7% - Easy to use, suitable for POC use with minimally trained healthcare professionals.
- Includes an internal procedural control line (C) to confirm proper execution.
Specifications:
Test kits include:
- 20 x test devices
- 20 x sterile swabs
- 2 x tube holders
- 1 x instruction for use
- 20 x pre-filled buffer tubes with flip-tops
- 1 x QRI for HealthCare Providers
- 1 x Self-swab collection instructions
- Specimen type: Nasal swab
- Storage: 2-30°C/36-86°F
- Shelf life: 24 months
Some of the SKUs are available for sale exclusively in the US. Please contact us for more information.
Ordering Information
| Product Description | Catalog No. | Kit Size |
|---|---|---|
| Healgen® Rapid COVID-19 Antigen Test | GCCOV-502a-NA | 20 Tests/Kit |
| COVID-19 Antigen Control Kit | GCCOV(Ag)-PN10 | 5 negative control swabs + 5 positive control swabs |
| GCCOV(Ag)-PN20 | 10 negative control swabs + 10 positive control swabs |

Healgen® Rapid Check™ COVID-19/ Flu A&B Antigen Test
For in vitro diagnostic use
For Over-the-Counter Use
The Healgen Rapid Check™ COVID-19/Flu A&B Antigen Test is a lateral flow immunochromatographic assay intended for the qualitative detection and differentiation of influenza A, and influenza B nucleoprotein antigens and SARS-CoV-2 nucleocapsid antigen directly in anterior nasal swab samples from individuals with symptoms of COVID-19 and influenza. Clinical signs
and symptoms of respiratory viral infection due to SARS-CoV-2 and influenza can be similar. This test is for non-prescription home use by individuals aged 14 years or older testing themselves, or adults testing individuals aged 2 years or older.
All negative results are presumptive and should be confirmed with an FDA-cleared molecular assay, if necessary for patient management. Negative results do not rule out infection with influenza, SARS-CoV-2 or other pathogens.
This test is not a substitute for visits to a healthcare provider and should not be used to determine any treatments without consulting with a healthcare provider. Individuals who test negative
and experience continued or worsening respiratory symptoms, such as fever, cough and/or shortness of breath, should seek follow up care from their healthcare provider.
Positive results do not rule out co-infection with other respiratory pathogens.
The Healgen Rapid Check™ COVID-19/Flu A&B Antigen Test is intended for non-prescription self-use and/or, as applicable, an adult lay user testing another person 2 years or older in a
non-laboratory setting
Features:
- Detect 3 viruses at once (SARS-CoV-2, Flu A&B)
- For symptomatic individuals within 5 days of symptom onset
- Shallow nasal swab sample collection
- Suitable for 2+ years old
* Swabbing should be performed by an adult for children aged 2 to 13. User must be aged 14 + to perform self test.
Specifications:
- Specimen: Nasal Swab
- Time to results: 15 minutes
- Storage: 36-86°F (2-30°C)
US market only. Coming soon for Canada market
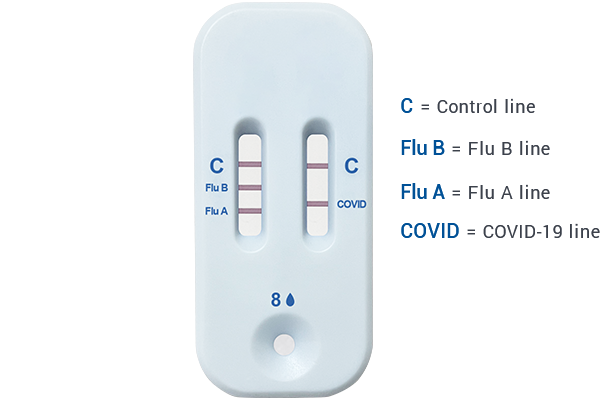
POSITIVE TEST RESULT
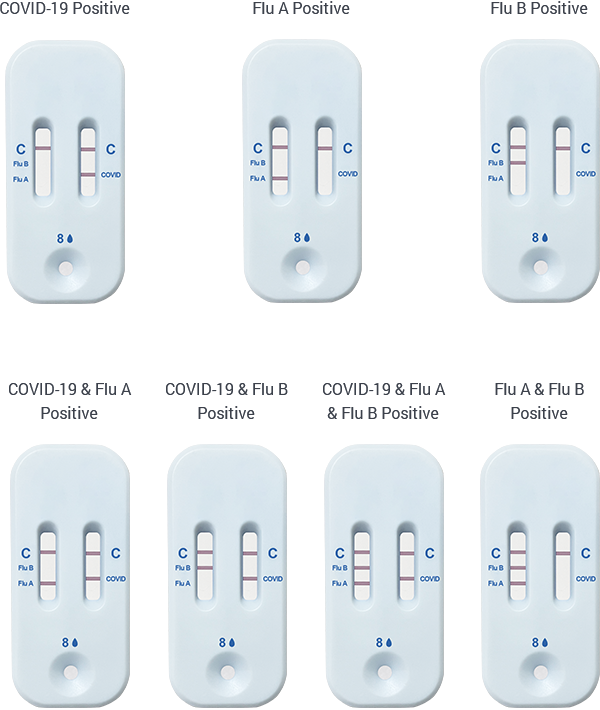
Both ‘C’ lines must be PRESENT
Consult your healthcare provider to discuss your positive test result.Self-isolate at home per CDC recommendations to stop spreading virus to others.
NEGATIVE TEST RESULT

Both ‘C’ lines only
If you do not see a line at ‘COVID’ , ‘Flu A’ or ‘Flu B’ , it means you may not have COVID-19 Flu A or Flu B virus.If you still have COVID-19,Flu A or Flu B symptoms,you should seek follow up care with your healthcare provider.
INVALID TEST RESULT
Missing ‘C’ line on ONE or BOTH strips
Check to see if a line is visible at the control line ‘C’ on both strips. If you do not see any C line, or only see one C line, DO NOT CONTINUE reading the results. It means your test is invalid. Repeat the test with a new sample and new test kit materials.
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size |
|---|---|---|---|---|
| COVID-19/ Flu A&B Antigen Test | Nasal Swab | GCFC-525SKa-Hx | Cassette | 1/2/4 Tests/kit |

COVID-19/Flu Combo
Some of the SKUs are available for sale exclusively outside the US. Please contact us for more information.

COVID-19/Flu/RSV Combo
Some of the SKUs are available for sale exclusively outside the US. Please contact us for more information.

COVID-19/Flu/RSV/Adeno Combo
Some of the SKUs are available for sale exclusively outside the US. Please contact us for more information.
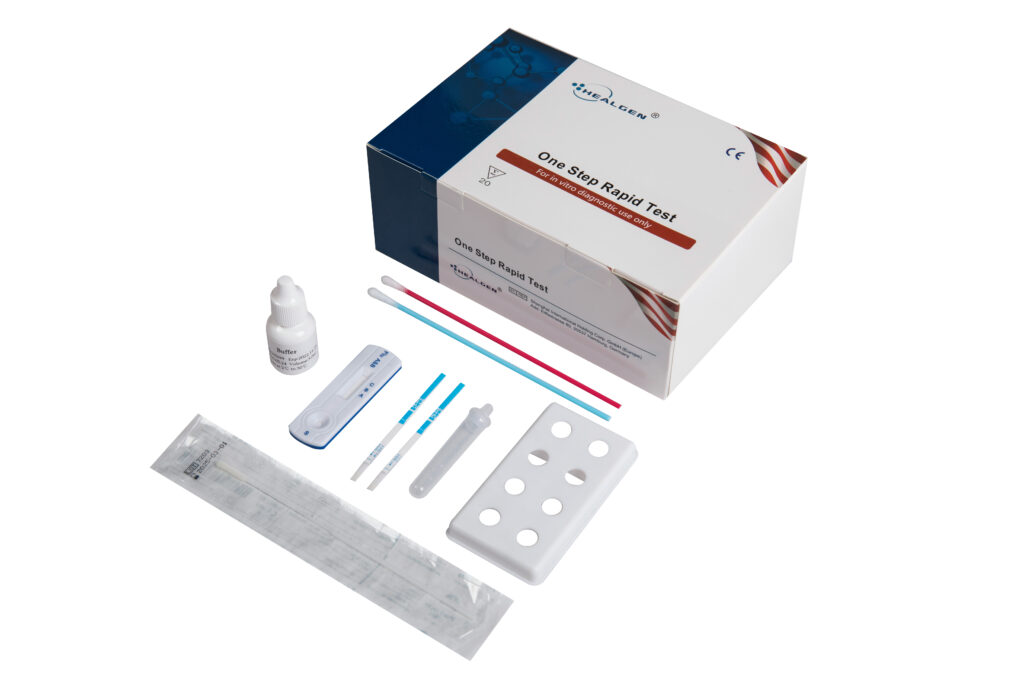
Influenza (Flu)
The Influenza Antigen Rapid Test is an in vitro immunochromatographic assay for the qualitative detection of influenza A (including the subtype H1N1) and/or B nucleoprotein antigens in nasopharyngeal (NP) swab, nasal swab, and nasal wash/aspirate specimens. It is intended to aid in the rapid differential diagnosis of influenza A and/or B viral infections.
Features:
- Two band results for simple interpretation
- Detects Influenza A/B antigen
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
Specifications:
- Cut-off:1.5 x 104TCID50/test for the Influenza A virus antigen;1.5 x 105TCID50/test for the Influenza B virus antigen
- Specimen: Nasal swab, Throat swab
- Time to Results: 10 minutes
- Shelf Life: 24 months from the date of manufacture
Some of the SKUs are available for sale exclusively outside the US. Please contact us for more information.
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Cut-off Value | Kit Size | Qualification |
|---|---|---|---|---|---|---|
| Influenza A Ag Rapid Test Strip | Nasal/Throat Swabs | GCFLU(A)-501a | Strip | 1.5×104 TCID50 | 25 Tests/Kit | CE marked |
| Influenza A Ag Rapid Test Cassette | Nasal/Throat Swabs | GCFLU(A)-502a | Cassette | 1.5×104 TCID50 | 20 Tests/Kit | CE marked |
| Influenza A&B Ag Rapid Test Strip | Nasal/Throat Swabs | GCFLU(A/B)-501a | Strip | 1.5×104 TCID50/ 1.5×105 TCID50 | 25 Tests/Kit | CE marked |
| Influenza A&B Ag Rapid Test Cassette | Nasal/Throat Swabs | GCFLU(A/B)-502a | Cassette | 1.5×104 TCID50/ 1.5×105 TCID50 | 20 Tests/Kit | CE marked |
| Influenza A&B Ag Rapid Test Cassette | Nasal/Throat Swabs | GCFLU(A/B)-502Ca | Cassette | 1.5×104 TCID50/ 1.5×105 TCID50 | 20 Tests/Kit | CE marked |
Mononucleosis
The Mononucleosis Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of 4 serologic markers of Epstein-Barr virus (EBV), two of IgM class, VCA and heterophile antibodies, and two of IgG class, VCA and EBNA, in human whole blood, serum or plasma. It is intended to be used as a screening test and as an aid in the diagnosis of infectious mononucleosis. Any reactive specimen with the Mononucleosis Rapid Test must be confirmed with alternative testing method(s) and clinical findings.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
- Internal control included
Specifications:
- For IgM: Sensitivity: 95.2%, Specificity: 98.3%
- For HA: Sensitivity: 96.9%, Specificity: 97.8%
- For EBNA-IgG: Sensitivity: 95.2%, Specificity: 96.3%
- For VCA-IgM: Sensitivity: 95.9%, Specificity: 97.6%
- For VCA-IgG: Sensitivity: 96.6%, Specificity: 97.5%
- Specimen: Whole blood, serum, plasma
- Time to Results: 8 to 15 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
| Mononucleosis IgM Rapid Test | Serum/Plasma | GCMON-325a | Cassette | 25 Tests/Kit | CE marked |
| Mononucleosis IgM Rapid Test | Whole blood/Serum/Plasma | GCMON-402a | Cassette | 25 Tests/Kit | CE marked |
| Mononucleosis IgG/IgM Rapid Test | Whole blood/Serum/Plasma | GCMON-425a | Cassette | 25 Tests/Kit | CE marked |
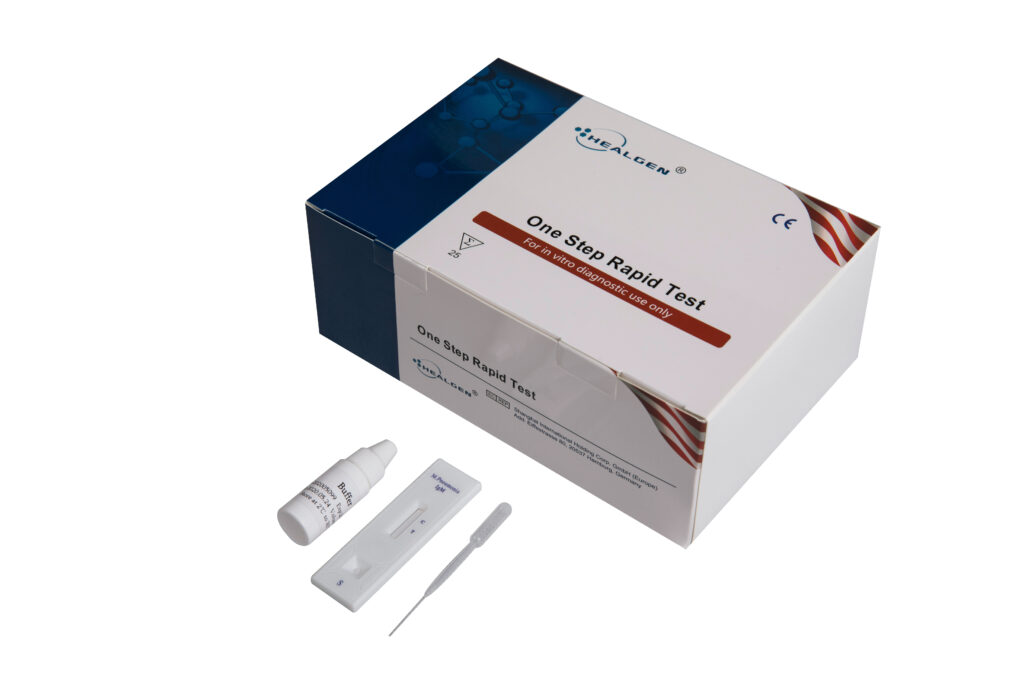
M. pneumoniae
The M. pneumoniae IgM Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of IgM antibodies to Mycoplasma pneumonia antigen in seru m or plasma to aid in the diagnosis of recent Mycoplasma pneumoniae infection.
Features:
- Detects M. pneumoniae IgM antibody
- Internal control included
- Reagents included
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
Specifications:
- Sensitivity: 96.4%
- Specificity: 96.3%
- Specimen: Serum, Plasma
- Time to Results: 5 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
| M. pneumoniae IgM Test | S/P | GCMP(IgM)-302a√ | Cassette | 25 Tests/Kit | CE marked |

RSV Antigen Test
The RSV Antigen Rapid Test cassette is rapid chromatographic immunoassay for the detection of
respiratory syncytial virus (RSV) antigen (viral fusion protein) in human nasal/nasopharyngeal swab, nasal/nasopharyngeal aspirate samples. It is intended as a as a screening test and as an aid in the
diagnosis of RSV respiratory infections.
Features:
- Health Canada Class II licensed.
- Rapid and accurate results within 15 minutes.
- Applicable for both nasal/nasopharyngeal swab, and nasal suction fluid use
Specifications:
- 20 tests/kit
- Storage: 2-30°C/36-86°F
- Shelf life: 24 months
Ordering Information
| Product Description | Catalog No. | Kit Size |
|---|---|---|
| RSV Antigen Rapid Test Cassette (Swab) | GCRSV-502a | 20 Tests/Kit |
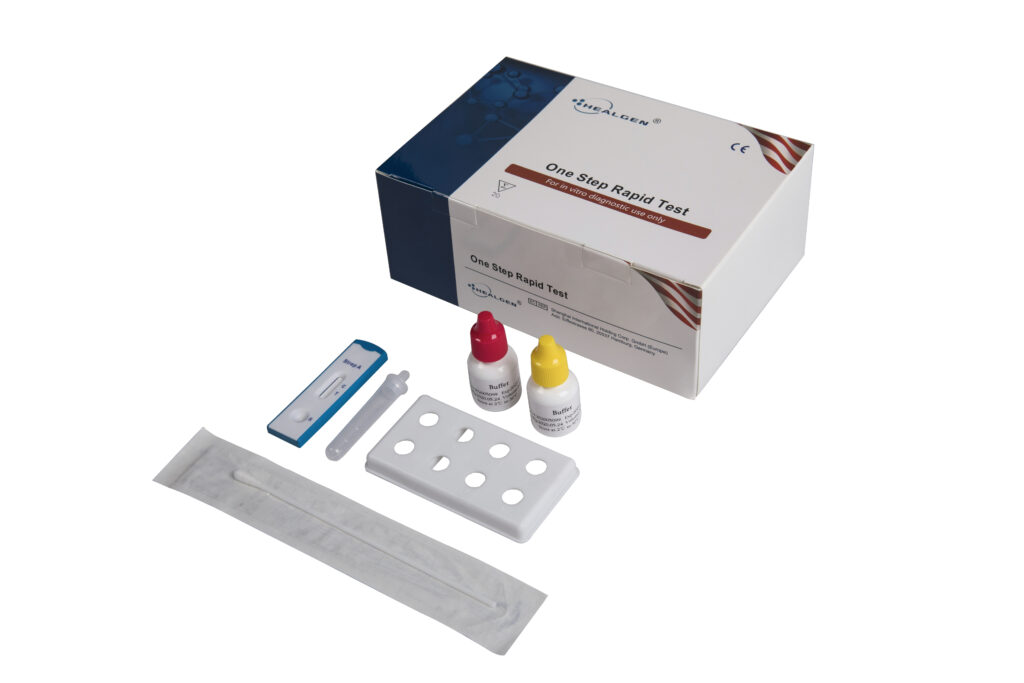
Strep A
The Strep A Rapid Test is a rapid chromatographic immunoassay for the qualitative detection of Strep A antigen from throat swab specimens to aid in the diagnosis of Group A Streptococcal infection.
Features:
- Two band results for simple interpretation
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
- Internal control included
Specifications:
- Sensitivity: 92.3%
- Specificity: 96.4%
- Specimen: Throat Swab
- Time to Results: 5 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
Strep A Rapid Test | Throat swab | GCSTR-501a√ | Strip | 25 Tests/Kit | CE marked |
| Throat swab | GCSTR-501Ca√╅ | Strip | 25 Tests/Kit | CE marked, 510(k) | |
| Throat swab | GCSTR-502a√ | Cassette | 20 Tests/Kit | CE marked | |
| Throat swab | GCSTR-502Ca√ | Cassette | 20 Tests/Kit | CE marked |
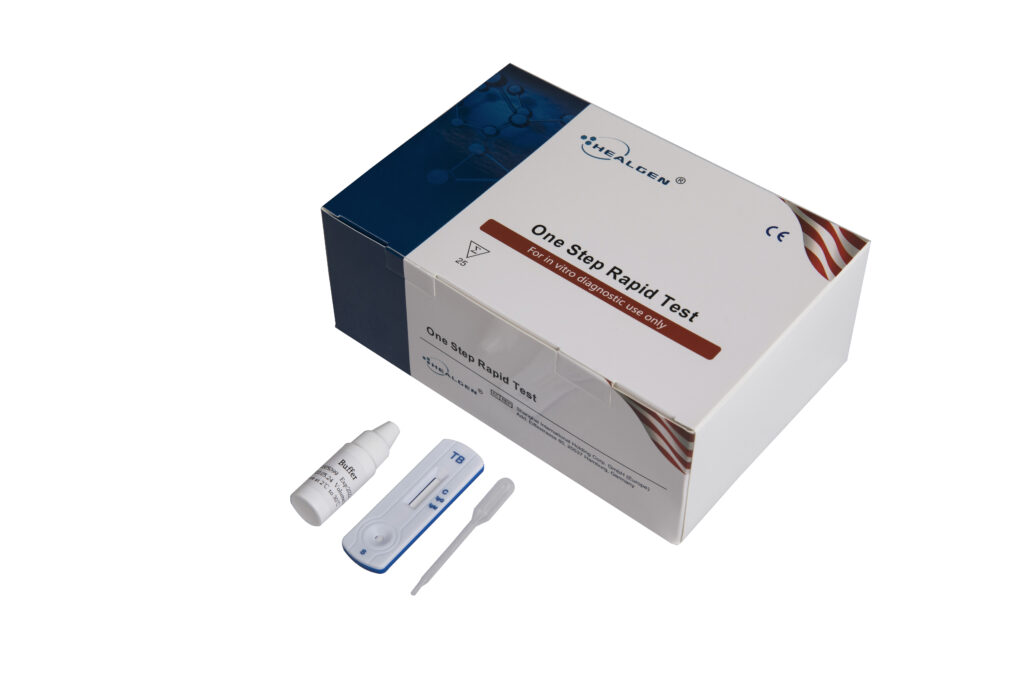
Tuberculosis
The Tuberculosis IgG/IgM Rapid Test is a rapid membrane-based screening test for the rapid detection of IgM anti-Mycobacterium Tuberculosis and IgG anti-Mycobacterium Tuberculosis in human in serum, plasma or whole blood.
Features:
- Two band results for simple interpretation
- Detects Tuberculosis antigen
- Room temperature storage or refrigerated (2-30⁰C)
- Easy to read results
Specifications:
- Clinical performance for IgG: Sensitivity: 87.2% / Specificity: 97.3% / Accuracy: 95.6%
- Clinical performance for IgM: Sensitivity: 83.5% / Specificity: 98.1% / Accuracy: 95.1%
- Specimen: Whole blood, Serum, Plasma
- Time to Results: 10 minutes
- Shelf Life: 24 months from the date of manufacture
Ordering Information
| Product Description | Specimen | Catalog No. | Format | Kit Size | Qualification |
|---|---|---|---|---|---|
Tuberculosis IgG/IgM Rapid Test | S/P | GCTB-302a√ | Cassette | 25 Tests/Kit | CE marked |
| WB/S/P | GCTB-402a√ | Cassette | 25 Tests/Kit | CE marked |